
On Mar. 28th, 2022, Chemical Science, the flagship journal of Royal Society of Chemistry, published a new edge research article on the dynamics of interfacial water at distinct materials entitled as “Universal Dynamical Onset in Water at Distinct Material Interfaces” by Prof. Liang Hong’s group (Institute of Natural Sciences/School of physics and astronomy), Prof. Wei Zhuang’ group (Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences) and Prof. He Cheng’ group (Dongguan Institute of Neutron Science) (DOI: 10.1039/D1SC04650K).
Bio-preservation is a key engineering process, crucial for the long-term storage and transportation of biomacromolecules (proteins, DNA, RNA, etc.), cells and living organs. Biomacromolecules exhibit a dynamical transition around -70 ℃, across which they transform from a rigid, harmonic state, to a flexible, anharmonic state. This transition is of direct connection to the functional onset of various biomacromolecules. The amorphous rigid state of biological systems below dynamical transition temperature can be stored for a long time, which rationalizes why the -80 ℃ Fridge are often used in various biological laboratories. In order to reduce the harsh requirement of cold chain on the biopreservation, one can add bio-compatible preservation agents (such as glycerol, trehalose, sucrose, etc.) in to the system to increase the preservation temperature. Therefore, the costs of storage and transportation can be reduced. For example, the initial biological preservation strategy of Pfizer's COVID-19 vaccine is to store mRNA at -60 ℃ by adding sucrose. Besides, protein drugs or key enzymes for nucleic acid detection can be stored for a long time at -20 ℃ or even room temperature by adding protective agents like sucrose, trehalose and glycerol etc. Increasing the storage temperature by adding the protective agents can significantly reduce the dependence of biological storage and transportation on the cold chain. Hence, studying of the microscopic mechanism underlying the dynamical transition and searching for the proper bio-preservation agent is not only of scientifically important but also of great engineering significance.
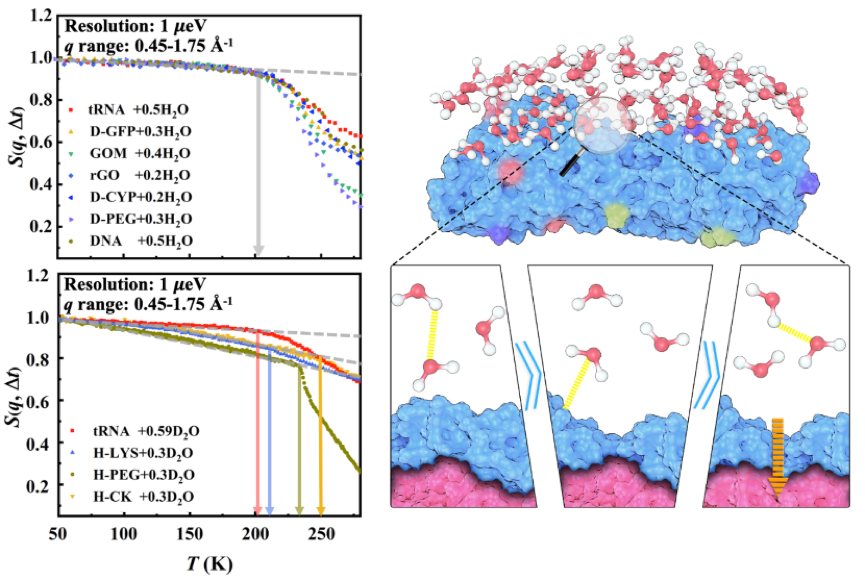
In the present work, the research group experimentally examined the temperature dependence of the dynamics of non-freezing interfacial water at subzero temperatures across a wide range of materials with drastically different chemical composition, structures and packing. In all the systems studied herein, the water molecules present a universal dynamical onset whose characteristic onset temperature is independent of the surface nature of the underlying materials as well as the level of hydration, but varies with the resolution of the neutron spectrometer used. The group provided the extensive experimental evidence over many different materials that the dynamical onset of interfacial water is an intrinsic property of water itself, resulting from a surface independent relaxation process in water with an approximately universal energy barrier of ~35 kJ/mol. Complementary computer simulations confirmed the experimental findings and revealed that this intrinsic relaxation corresponds to the switching of hydrogen bond between neighboring hydration water molecules. Moreover, they found that, although the fast relaxation of the materials has the same energy barrier as its surface water, its rate is strongly dependent on their structure, packing and interaction with the surface water. The latter leads to system-dependent dynamical onsets and different flexibilities at low temperatures, which could be crucial for their functions at subzero temperatures. More importantly, this work not only reveals the general mechanisms behind the dynamic transitions that prevail in biological systems, but also provides new strategy for the development of new biological cryo-preservations: tuning the chemical structure of the preservation agent to change the dynamics of hydrogen bonds between solvent molecules and then to optimize the preservation temperature.
This is a collaborative work by Prof. Liang Hong at SJTU, Prof. Wei Zhuang at Fujian Institute of Research on the Structure of Matter and Prof. He Cheng at China Spallation Neutron Source. The first author is Mr. Lirong Zheng, the PhD student in Liang’s group. The work was supported National Natural Science Foundation of China and Shanghai Municipal Scienwce and Technology Major Project. The beam time was supported by Madhusudan Tyagi from NIST (US), Victoria García Sakai from ISIS (UK) and Takeshi Yamada from J-Parc (Japan).




